Ongoing projects:
1. Develop a mouse model and a gene therapy for MTATP6 Leigh syndrome
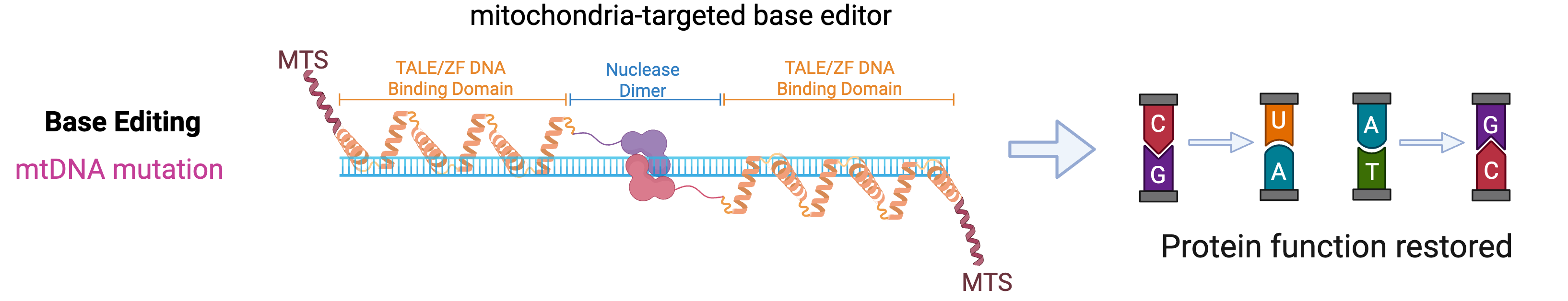
We are developing the first disease-relevant mouse model and an AAV-based gene therapy for mitochondrial DNA-encoded ATPase 6 (MTATP6)-related mitochondrial diseases. Mutations in MTATP6 are the most common cause of impaired mitochondrial ATP synthesis, leading to neurological mitochondrial diseases. There are currently no approved treatments for any of its disease forms. Unlike the vast majority of genes that are being targeted for gene therapy, the MTATP6 gene is encoded in the mitochondrial genome, which poses greater challenges.
2. Develop a gene therapy for NF2-related schwannomatosis
Neurofibromatosis type 2-related schwannomatosis (NF2-SWN) is a rare, inherited tumor predisposition syndrome characterized by the development of multiple benign tumors of the nervous system, particularly bilateral vestibular schwannomas. It is caused by loss-of-function mutations in the NF2 gene, which encodes the tumor suppressor protein MERLIN. We are developing a gene therapy for this devastating disease.

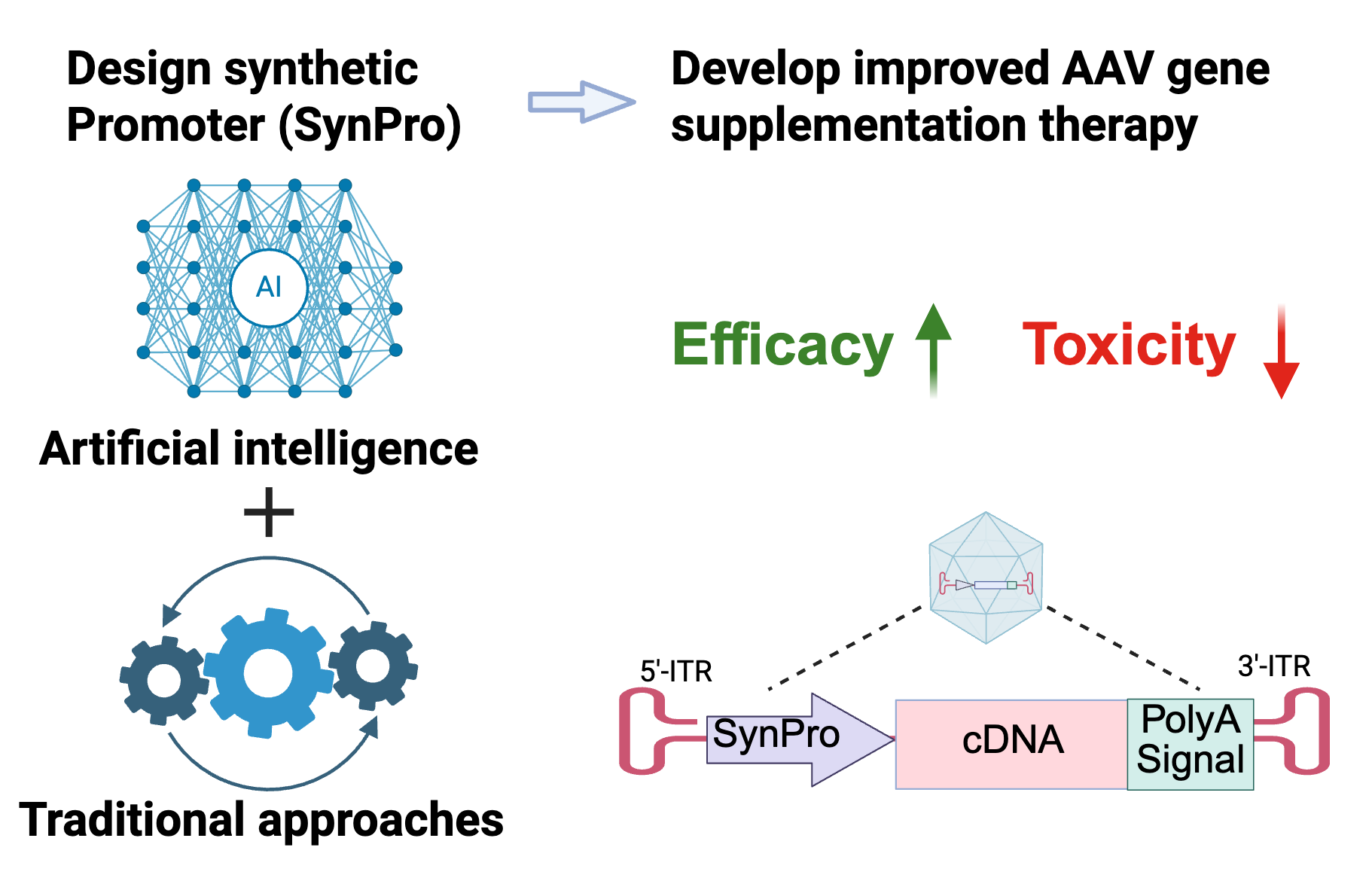
3. Develop a metabolic state-responsive gene therapy by engineering synthetic promoters
This project aims to address the limitations of current gene supplementation therapy by designing a SMART gene therapy, which Senses the Metabolic state of transduced cells and Achieves the “just Right” level of Therapeutic transgene expression. Adeno-associated virus (AAV)-based gene supplementation therapy is a promising therapeutic option for genetic diseases with protein loss-of-function or deficiency evident by numerous recent clinical trials. However, regulating the expression of transgenes in a cell-specific and dynamic manner is challenging. Our lab aims to address this issue by engineering synthetic promoter (SynPro) sequences that are responsive to the metabolic states of cells. We will leverage advancements in bioinformatics analysis of single-cell transcriptomics and epigenomics data acquired from either publicly available datasets, or genetic engineered human cell lines. Successfully establishing this platform can significantly improve the safety, efficacy, as well as shortening the timeline related to the development of gene therapies for these and other diseases that are associated with metabolic stress, including cancer, Type 2 diabetes and Alzheimer’s disease.